Abstract
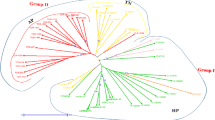
In the present investigation, cross-transferability of 125 B. rapa specific and 70 B. nigra specific genomic-SSRs had been evaluated in 16 genotypes of B. carinata (Ethiopian mustard) with an aim to develop genomic resources for Ethiopian mustard. A cross-transferability rate of 82.4% (103 SSRs) and 84.3% (59 SSRs) had been obtained for B. rapa- and B. nigra- derived SSRs, respectively, out of which 66 (64.1%) of B. rapa- and 37 (62.7%) of B. nigra- SSRs resulted into polymorphic amplicons. Comparison of polymorphism parameters including PIC value, gene diversity and heterozygosity values revealed that B. nigra-SSRs were more efficient in terms of transferability rate and polymorphic potential than B. rapa-SSRs. Unweighted pair group method with arithmetic averages-dendrogram divided Ethiopian mustard genotypes into four groups. This newly characterized set of SSR markers will enrich the repertoire of B. carinata SSRs and would provide new paradigms for B. carinata genomics-based improvement.

Similar content being viewed by others
Availability of data and materials
All the data and plant material is available with the corresponding author (KHS).
References
Agarwal, M., Shrivastava, N., & Padh, H. (2008). Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Reports, 27, 617–631.
Atri, S., Sharma, S., & Banga, S. S. (2018). Genome specific microsatellites in wild crucifers: Cross species/genera transferability. Indian Journal of Biotechnology, 17, 80–90.
Chauhan, J. S., Singh, K. H., Singh, V. V., & Kumar, S. (2011). Hundred years of rapeseed-mustard breeding in India: Accomplishments and future strategies. Indian Journal of Agricultural Sciences, 81, 1093–1109.
Getinet, A., Rakow, G., & Downey, R. K. (1996). Agronomic performance and seed quality of Ethiopian mustard in Saskatchewan. Canadian Journal of Plant Science, 76, 387–392.
Gruppen, G. J. H., & Denton, O. A. (2004). Brassica carinata. Prota 2 Vegetables (pp. 119–123). Backhuys Publication.
Guo, S., Zou, J., Li, R., Long, Y., Chen, S., & Meng, J. (2012). A genetic linkage map of Brassica carinata constructed with a doubled haploid population. Theoretical and Applied Genetics., 125, 1113–1124.
Hopkins, C. J., Cogan, N. O. I., Hand, M., Jewell, E., Kaur, J., Li, X., et al. (2007). Sixteen new simple sequence repeat markers from Brassica juncea expressed sequences and their cross-species amplification. Molecular Ecology Notes, 7, 697–700.
Kalia, R. K., Rai, M. K., Kalia, S., Singh, R., & Dhawan, A. K. (2011). Microsatellite markers: An overview of the recent progress in plants. Euphytica, 177, 309–334.
Katiyar, R. K., Saran, G., & Giri, G. (1986). Evaluation of Brassica carinata as a new oilseed crop in India. Experimental Agriculture, 22, 67–70.
Liu, K., & Muse, M. (2005). PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics, 21, 2128–2129.
Malik, R. S. (1990). Prospects for Brassica carinata as an oilseed crop in India. Experimental Agriculture, 26, 125–130.
Márquez-Lema, A., Velasco, L., & Pérez-Vich, B. (2010). Transferability, amplification quality, and genome specificity of microsatellites in Brassica carinata and related species. J App Genet, 51, 123–131.
Mendlinger, S., Chadha, M. L., Oluoch, M., & Volis, S. (2006). Germplasm collection, evaluation and improvement of African leafy vegetables. Report to USAID TA-MOU-C21-054.
Nagaharu, U. (1935). Genomic analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Journal of Japanese Botany, 7, 89–452.
Nanjundan, J., Singh, K., Singh, K. H., & Singh, D. (2014). Catalogue on rapeseed-mustard germplasm. Directorate of Rapeseed-Mustard Research, Bharatpur, Rajasthan (p. 180).
Phan, H. T., Ellwood, S. R., Hane, J. K., Ford, R., Materne, M., & Oliver, R. P. (2007). Extensive microsynteny between Medicago truncatula and Lens culinaris ssp. culinaris. Theoretical and Applied Genetics, 114, 549–558.
Plieske, J., & Struss, D. (2001). Microsatellite markers for genome analysis in Brassica. I. Development in Brassica napus and abundance in Brassicaceae species. Theoretical and Applied Genetics, 102, 689–694.
Rakow, G. (1995). Developments in the breeding of edible oil in other Brassica species. In Proceedings of the 9th international rapeseed conference, 4–7 July, 1995, Cambridge, UK (pp. 401–406).
Sharma, B. B., Kalia, P., Singh, D., & Sharma, T. R. (2017). Introgression of black rot resistance from Brassica carinata to cauliflower (Brassica oleracea botrytis group) through embryo rescue. Frontiers in Plant Science, 8, 1255.
Sheikh, F. A., Najeeb, S., Rather, A. G., & Banga, S. (2010). Resynthesis of Ethiopian mustard (Brassica carinata L.) from related digenomic species: An unexplored possibility. Journal of Agricultural Biotechnology and Sustainable Development, 2, 30–34.
Simmonds, N. W. (1979). Principles of crop improvement. Longman.
Singh, H., Singh, D., & Yadava, T. P. (1988). Comparative performance of the genotypes of Indian and Ethiopian mustard under semi-arid region of India. Cruciferae Newslett, 13, 36–37.
Singh, K. H., Shakya, R., Singh, K. K., Thakur, A. K., Nanjundan, J., & Singh, D. (2015). Genetic enhancement of Brassica carinata through interspecific hybridization and population improvement. In 14th international rapeseed congress (p. 281).
Teklewold, A., & Becker, H. C. (2006). Geographic pattern of genetic diversity among 43 Ethiopian mustard (Brassica carinata A. Braun) accessions as revealed by RAPD analysis. Genetic Resources and Crop Evolution, 53, 1173–1185.
Thakur, A. K., Singh, K. H., Lal, S., Nanjundan, J., Yasin, J. K., & Singh, D. (2018). SSR marker variations in Brassica species provide insight into the origin and evolution of Brassica amphidiploids. Hereditas, 155, 6.
Thakur, A. K., Singh, K. H., Singh, L., Nanjundan, J., Rana, M. K., & Singh, D. (2015). Transferability of SSR markers of Brassica species to some popular varieties of Brassica juncea. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 85, 1001–1010.
Thakur, A. K., Singh, B. K., Verma, V., & Chauhan, J. S. (2013). Direct organogenesis in Brassica juncea var. NRCDR-2 and analysis of genetic uniformity using RAPD markers. National Academy Science Letters, 36, 403–409.
Tsunoda, S. (1980). Eco-physiology of wild and cultivated forms in Brassica and allied genera. In S. Tsunoda, K. Hinata, & C. Gómez-Campo (Eds.), Brassica crops and wild allies (pp. 109–120). Japan Scientific Societies Press.
Warwick, S. I. (2011). Brassicaceae in agriculture. In R. Schmidt & I. Bancroft (Eds.), Genetics and genomics of the Brassicaceae (pp. 33–65). New York: Springer.
Warwick, S. I., Gugel, R. K., McDonald, T., & Falk, K. C. (2006). Genetic variation of Ethiopian mustard (Brassica carinata A. Braun) germplasm in western Canada. Genetic Resources and Crop Evolution, 53, 297–312.
Wei, Z., Wang, M., Chang, S., Wu, C., Liu, P., Meng, J., et al. (2016). Introgressing subgenome components from Brassica rapa and B. carinata to B. juncea for broadening its genetic base and exploring intersubgenomic heterosis. Frontiers in Plant Science, 7, 1677.
Xiao, Y., Chen, L., Zou, J., Tian, E., Xia, W., & Meng, J. (2010). Development of a population for substantial new type Brassica napus diversified at both A/C genomes. Theoretical and Applied Genetics, 121, 1141–1150.
Xu, J., Qian, X., Wang, X., Li, R., Cheng, X., Yang, Y., Fu, J., Zhang, S., King, G. J., Wu, J., & Liu, K. (2010). Construction of an integrated genetic linkage map for the A genome of Brassica napus using SSR markers derived from sequenced BACs in B. rapa. BMC Genomics, 11, 594.
Yadava, D. K., Parida, S. K., Dwivedi, S. K., Varshney, A., Ghazi, I. A., Sujata, V., & Mohapatra, T. (2009). Cross-transferability and polymorphic potential of genomic STMS markers of Brassica Species. Journal of Plant Biochemistry and Biotechnology, 18, 29–36.
Funding
The authors wish to thank Indian Council of Agricultural Research (ICAR), New Delhi for funding this work under ICAR-Extra-Mural Research Grant Scheme.
Author information
Authors and Affiliations
Contributions
AKT & KHS formulated the research methodology; DS, NP, LS & JN prepared the draft; DCM performed the data analysis, and AKT & KHS edited and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thakur, A.K., Singh, K.H., Sharma, D. et al. Enriching the repertoire of SSR markers of Ethiopian mustard using cross-transferability approach. Plant Physiol. Rep. 27, 65–72 (2022). https://doi.org/10.1007/s40502-021-00639-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-021-00639-4